Why we lose NAD+ in aging [DNA repair & inflammation] | David Sinclair
Get the full length version of this episode as a podcast.
This episode will make a great companion for a long drive.
The Omega-3 Supplementation Guide
A blueprint for choosing the right fish oil supplement — filled with specific recommendations, guidelines for interpreting testing data, and dosage protocols.
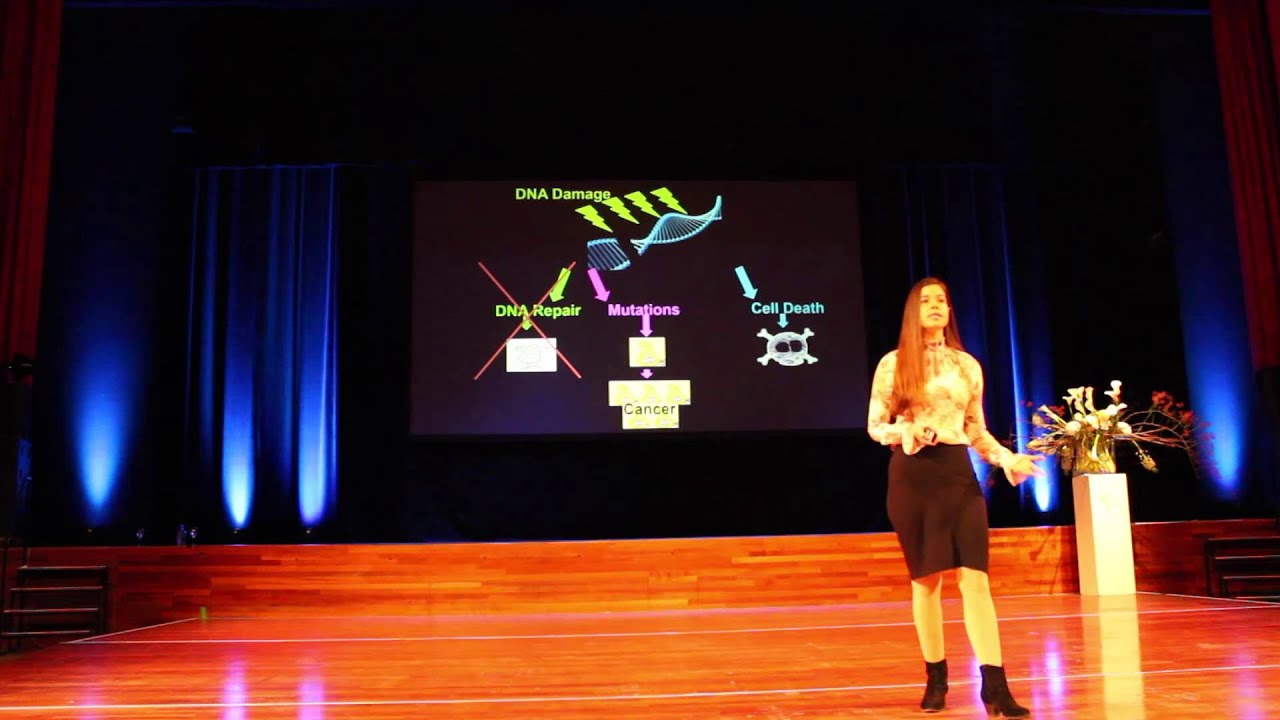
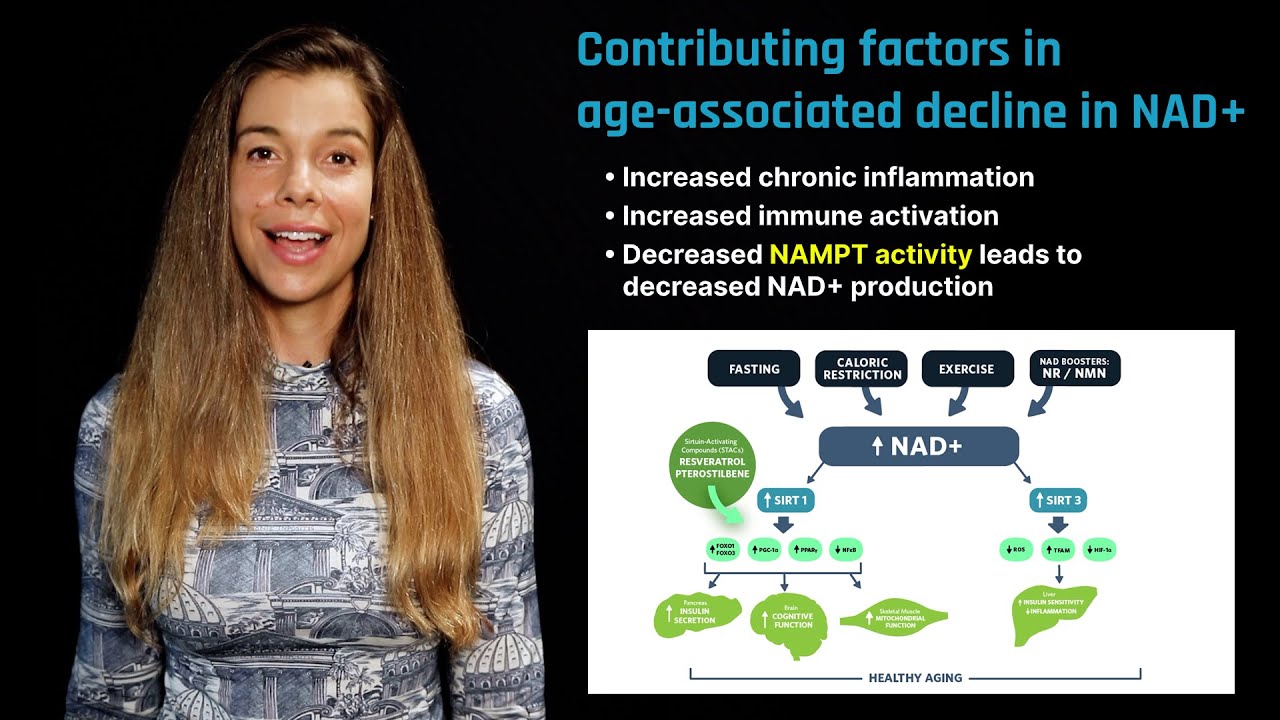
As we age, immune system activation and increased levels of DNA damage utilize much of the body's NAD+. The body also produces less NAD+ over time, creating a vicious cycle of increased need and decreased production. This can lead to genomic instability – a wide range of alterations that occur in our DNA and irreversibly change the information carried in our genome. Sirtuins respond quickly to DNA damage within a cell, directing gene expression to promote DNA repair. This serves as a distraction to sirtuins, however, ultimately drawing them away from their primary role. In the end, the cell experiences a sort of "identity crisis," unable to perform its original job, leading to aging. In this clip, Dr. David Sinclair explains why NAD+ levels decrease with age.
Rhonda: Let me ask you this. This is kind of something that comes to my mind. I don't think it's often thought about this way, at least, in the field. But, you know, because NAD is required for cells that have a really high energetic demand like activated immune cells, for example, activated immune cells require a lot of ATP for energy and a lot of NAD. And if you think about like chronic inflammation, how, you know, especially as you get older, and you're unhealthy, and with age, you know, basically it is increasing. If you're having more activated immune cells, is there any way to test if, like, the NAD, like there's a triage where NAD is kind of being sucked away to these activated immune cells and, like, then your mitochondria are now suffering and you get, like, mitochondrial decay because you're sort of shunting all this NAD to, like, take care of, you know, what your body thinks is potentially an infection that could kill you, right? So there's probably some sort of evolutionary mechanisms at play that say, "Oh, yeah, immune cells need this NAD more than mitochondria," or something, I don't know. So it'd be kind of interesting. Do you follow what I'm saying where...?
David: Oh, I absolutely do. I think you're probably right in thinking along the same lines that as you get older, you're losing the ability to make NAD, but you're also chewing it up. And as it gets worse and worse as you get older, the immune system is a big drain on NAD, and actually, so is DNA repair with the activation of PARPs. And once you drain NAD a little bit, then your PARPs and your immune system won't work, but then they'll need more NAD because you'll get more damage. And this is a positive feedback in a bad way so that once you start going down the NAD decline, the cells just start to need more, and more, and more with accumulating DNA damage. And that's actually what happens in yeast cells, going back to those little critters that we found that they became overwhelmed with damaged DNA and the sirtuins were overwhelmed, they had to go over and repair that genomic instability, the DNA instability. And one of the reasons old cells became sterile, which is a hallmark of yeast aging, is because the sirtuins are keeping the cells fertile back here, but they're so distracted by all this other DNA damage that's going on over here that they lose their identity. And that's a theme that we've discovered is likely true in mammals as well, that accumulations of DNA damage distract the sirtuins from their normal job of keeping a cell with the proper gene expression, and cellular identity and we see the loss of cellular identity over time in mice, at least. And what we're trying to do is to raise NAD levels back up so that they can fix the DNA damage, but also get back to where they came from and make sure the cell doesn't lose its identity too much.
Rhonda: I didn't know sirtuins played a direct role, and I guess they're regulating so many genes that they're playing a role in DNA repair and DNA damage.
David: Well, they're one of the first proteins to get to a broken chromosome.
Rhonda: Really?
David: Yeah, we discovered that. It's a while ago, it was a cell paper in 1999, if anyone would like to look it up. Mills, myself, and Guarente published that. SIR2 goes to a broken DNA end and then helps recruit other proteins.
Rhonda: A single-strand break or...?
David: Double.
Rhonda: Double? Really? So, like gamma-H2AX?
David: Yeah. So the first thing that happens is gamma-H2AX gets lit up on the break and then within seconds, SIRT1 brings in HDAC1, helps remodel the DNA in the chromatin so that it's ready for the repair proteins to come in and without SIRT1 getting there, these other repair proteins are very inefficient...
Rhonda: Interesting, I didn't know that.
David: ...but they're distracted. Sirtuins should actually be regulating genes elsewhere.
Rhonda: Right. Wow. That's really important to know..
David: Right. This is all part of my idea, my hypothesis called the information theory of aging, is that we're really losing the information regulation over time and all of these other things that occur such as telomere loss, and mitochondria loss, and loss of proteostasis, as Andy would call it, loss of protein folding mechanisms, this could be upstream of all of that, that our cells lose their identity and don't turn on the right genes the way they did when we were young. But the trick is how do you get everybody to go back and reset? And that's what we've been working on.
An energy-carrying molecule present in all cells. ATP fuels cellular processes, including biosynthetic reactions, motility, and cell division by transferring one or more of its phosphate groups to another molecule (a process called phosphorylation).
A tightly coiled molecule of DNA found in the nucleus of a cell. Chromosomes contain the genes and other genetic material for an organism. Humans have 46 chromosomes arranged in 23 pairs. Each chromosome is comprised of long stretches of DNA wrapped around proteins called histones, which provide structural support. At the end of each chromosome is a repetitive nucleotide sequence called a telomere. Telomeres form a protective “cap” – a sort of disposable buffer that gradually shortens with age – that prevents chromosomes from losing genes or sticking to other chromosomes during cell division.
A major contributing factor to aging, cellular senescence, and the development of cancer. Byproducts of both mitochondrial energy production and immune activity are major sources of DNA damage. Additionally, environmental stressors can increase this base level of damage. DNA damage can be mitigated by cellular repair processes; however, the effectiveness of these processes may be influenced by the availability of dietary minerals, such as magnesium, and other dietary components, which are needed for proper function of repair enzymes.
The phosphorylated version of histone 2A that forms when double-strand breaks in DNA occur. Formation of gamma-H2AX acts as a signal for DNA repair enzymes to be recruited to the site of damage in order to repair it. Gamma-H2AX is a biomarker for DNA damage.
The process in which information stored in DNA is converted into instructions for making proteins or other molecules. Gene expression is highly regulated. It allows a cell to respond to factors in its environment and involves two processes: transcription and translation. Gene expression can be turned on or off, or it can simply be increased or decreased.
A critical element of the body’s immune response. Inflammation occurs when the body is exposed to harmful stimuli, such as pathogens, damaged cells, or irritants. It is a protective response that involves immune cells, cell-signaling proteins, and pro-inflammatory factors. Acute inflammation occurs after minor injuries or infections and is characterized by local redness, swelling, or fever. Chronic inflammation occurs on the cellular level in response to toxins or other stressors and is often “invisible.” It plays a key role in the development of many chronic diseases, including cancer, cardiovascular disease, and diabetes.
A concept based on the assumption that the loss of function that accompanies aging is due to impaired cellular repair mechanisms caused by the accumulation of genetic damage in the cells.
Tiny organelles inside cells that produce energy in the presence of oxygen. Mitochondria are referred to as the "powerhouses of the cell" because of their role in the production of ATP (adenosine triphosphate). Mitochondria are continuously undergoing a process of self-renewal known as mitophagy in order to repair damage that occurs during their energy-generating activities.
A family of proteins involved in a number of cellular processes such as DNA repair, genomic stability, and programmed cell death (apoptosis). PARP's primary role is to detect and initiate an immediate cellular response to metabolic, chemical, or radiation-induced single-strand DNA breaks by signaling the enzymatic machinery involved in repair. NAD+ is required as substrate for generating ADP-ribose monomers. Evidence suggests that overactivation of PARP may deplete cellular stores of NAD+.
A portmanteau of the words protein and homeostasis. Proteostasis is maintained through the competing and integrated biological pathways within cells that control the biogenesis, folding, trafficking and degradation of proteins present within and outside the cell. Proteostasis deteriorates with age. As a result, the prevalence of age-related protein misfolding diseases, such as Alzheimer’s disease and Parkinson’s disease, increases.
A member of the sirtuin protein family. SIRT1 is an enzyme that deacetylates proteins that contribute to cellular regulation (reaction to stressors, longevity). It is activated by the phytochemical resveratrol as well as fasting.
A class of enzymes that influence that influence aging and longevity through multiple molecular pathways. Sirtuins regulate a variety of metabolic processes, including release of insulin, mobilization of lipids, response to stress, and modulation of lifespan. They also influence circadian clocks and mitochondrial biogenesis. Sirtuins are activated when NAD+ levels rise. The dependence of sirtuins on NAD+ links their enzymatic activity directly to the energy status of the cell via the cellular NAD+:NADH ratio, the absolute levels of NAD+, NADH or nicotinamide or a combination of these variables. There are seven known sirtuins, designated as Sirt1 to Sirt7.
Distinctive structures comprised of short, repetitive sequences of DNA located on the ends of chromosomes. Telomeres form a protective “cap” – a sort of disposable buffer that gradually shortens with age – that prevents chromosomes from losing genes or sticking to other chromosomes during cell division. When the telomeres on a cell’s chromosomes get too short, the chromosome reaches a “critical length,” and the cell stops dividing (senescence) or dies (apoptosis). Telomeres are replenished by the enzyme telomerase, a reverse transcriptase.
Member only extras:
Learn more about the advantages of a premium membership by clicking below.
Supporting our work
If you enjoy the fruits of
 , you can participate in helping us to keep improving it. Creating a premium subscription does just that! Plus, we throw in occasional member perks and, more importantly, churn out the best possible content without concerning ourselves with the wishes of any dark overlords.
, you can participate in helping us to keep improving it. Creating a premium subscription does just that! Plus, we throw in occasional member perks and, more importantly, churn out the best possible content without concerning ourselves with the wishes of any dark overlords.