Jed Fahey discusses the longevity compound Sulforaphane
Enter your email to get our 15-page guide to sprouting broccoli and learn about the science of chemoprotective compount sulforaphane.
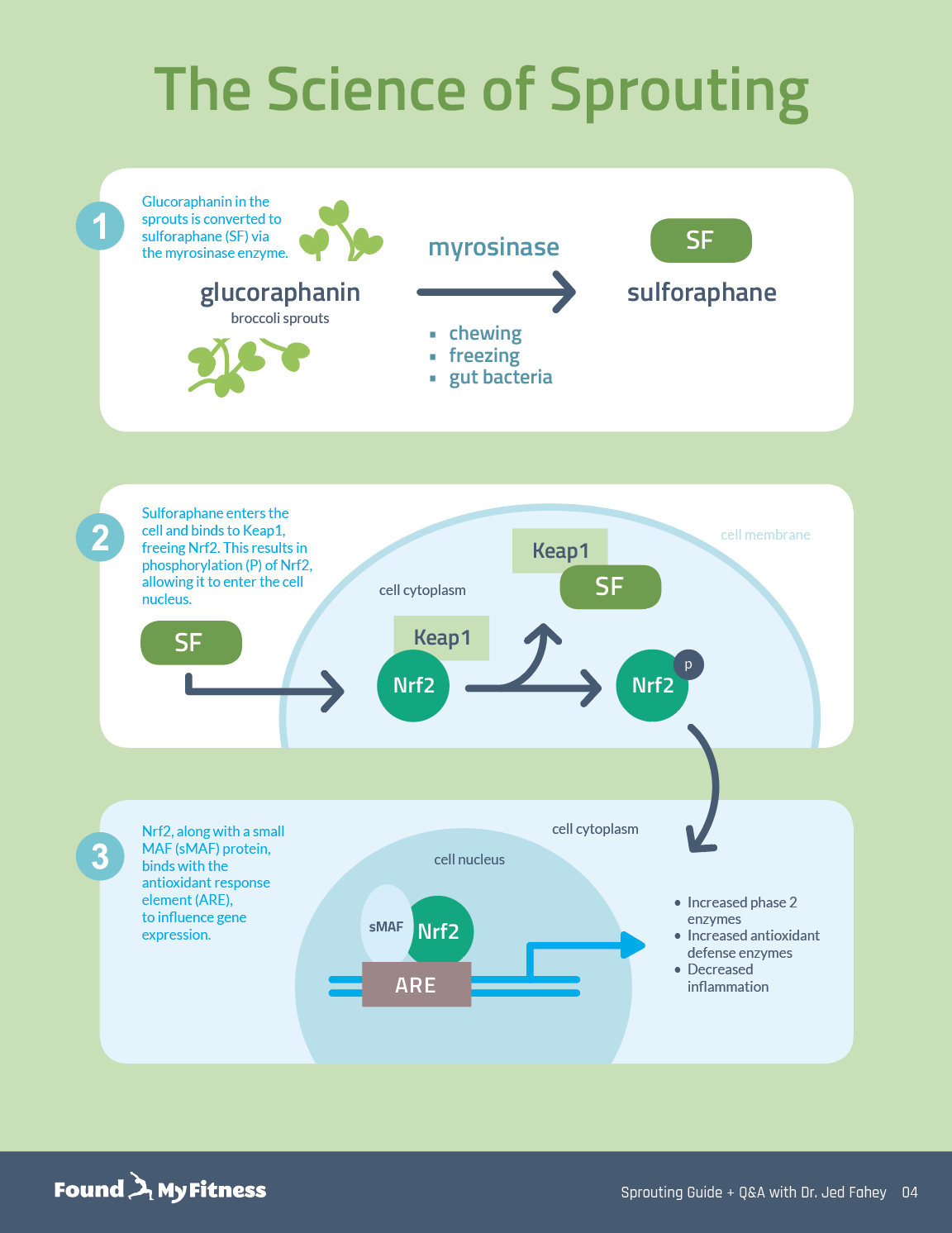
Broccoli sprouts are concentrated sources of sulforaphane, a type of isothiocyanate. Damaging broccoli sprouts – when chewing, chopping, or freezing – triggers an enzymatic reaction in the tiny plants that produces sulforaphane.
Get the full length version of this episode as a podcast.
This episode will make a great companion for a long drive.
Sulforaphane switches on the activity of a myriad of cytoprotective mechanisms, including Nrf2, a cellular protein that regulates the expression of antioxidant and stress response proteins. These proteins provide protection against oxidative stress due to injury and inflammation – key players in the aging process. Studying centenarians (people who live to the age of 100 years or more) might provide insights into whether sulforaphane confers longevity benefits through its actions on Nrf2. In this clip, Dr. Jed Fahey describes the current state of the science of understanding how sulforaphane influences longevity.
Jed: So there are collections of people who have made it to the ripe old age of 100 and more. Aren't they a beautiful cohort to look at the Nrf2-induced genes in? And I would be surprised if this hasn't been done already. We've talked about it for years, and I know we've talked about doing it in animals. But doing precisely, as you suggest, here is a cohort of very old people who are quite healthy. Do they have some... You know, everybody wants to know is there something magic about that? But are the Nrf2-inducible cytoprotective genes part of that equation? And we suspect that they might be. The prolonged lifespan experiments will pretty much have to be done in mice. And again, there is someone at the...there's a colleague that I know we've talked to at the Institute of Aging who was interested in doing that. I'm not sure if that ever happened. I had a high school student who wanted to do a project here a number of years ago and we got her working on nematodes, Caernorhabditis, which is a common experimental vehicle for those interested in looking at lifespan because its lifespan is so short and it cycles quickly.
Rhonda: I did a lot of work on C. elegans when I was...before I went to grad school, lifespan studies.
Jed: Well, we ought to get together and do this experiment again if it hasn't been done. You know, one of the problems with sulforaphane research nowadays is I can't keep up with it all. No one can really keep it up with it all because there are two or three papers a week coming out on sulforaphane and quite a few more on the Nrf2 pathway. I mean, if you try to be a renaissance person and know all the literature in this field, it's going to smoke you because there are thousands, there are, I think, a couple of thousands of papers a year on Nrf2. So it's very difficult to keep track of them all and it's possible that someone has done those lifespan experiments with C. elegans. Again, we started, we didn't have much muscle in it. We had a high school student who then had to go back to this...
Rhonda: Any preliminary data? I didn't see anything in the literature, by the way. I looked for C. elegans. I didn't see anything with sulforaphane that was published.
Jed: No, we don't have any preliminary data from that student. We also had, I'm looking at my shelf for the binder. It may not still be here. We had a colleague who was a bona fide expert on C. elegans who was going to do some studies and I'm not sure if that ever happened or if those were ever published. So, yeah, sorry, I wish I could jump up and down with great...
Rhonda: But do you agree with me here? I mean, just knowing what we know about how sulforaphane is, as you mentioned, the most potent dietary, that we know of activator, of the Nrf2 system, and knowing what we know, we do know that Nrf2 does play a role in delaying aging, in brain aging, in tissue aging. It's literally lowering inflammation and reducing oxidative stress. These things cause aging.
Jed: Right. Many people seem to think that by understanding a bit more about how we can reverse this phenotype in people with acute aging, that this may also be applicable to normal aging and to perhaps slowing down normal aging. It turns out that all of us have some small amount of progerin, this protein in our systems. And I believe the evidence is that it increases somewhat as we age, not certainly to the acute levels that you find in kids with progeria.
So we're using sulforaphane to explore this. Since we got our grant, there's a very interesting and, I think, quite important publication from Tom Misteli's group at the NIH showing that, in fact, Nrf2 is very intimately related to this process in progeria. And that what happens is that progerin, the sticky protein that I told you is on the inside of the nucleus, actually binds Nrf2 as it enters the nucleus to do its signaling thing and it gloms it up against the inside of the nucleus, so to speak, so that perhaps one among the chores of sulforaphane or among the functions of sulforaphane are to increase the amount of Nrf2 that's coming into the nucleus, certainly, but it may also enhance clearance of mutant proteins and even progerin from the nucleus. So there's a huge amount that we don't know there, but it's an exciting...
Rhonda: Very exciting.
Jed: It's been exciting for us just studying aging.
Rhonda: Yeah. It sounds like even just the mutant progerin would, seems like it's stopping Nrf2 from being activated in that...
Jed: It's sort of holding it up at the gate.
Rhonda: ...essentially, it's stopping it, right. And so that's possibly part of the pro-aging effect, as well. I was reading somewhere, maybe you can confirm this, that sulforaphane does increase the activity of Nrf2, like, Nrf2, I think it's something like 60%. Like, Nrf2 is activated every 180 minutes or something like that. And sulforaphane bumps that up a 60% increase in it being activated. So I don't know if that's...
Jed: Yeah, I can't comment on that. Sorry. But I mean...
Rhonda: But it's activating it more often for sure?
Jed: Correct, correct. Yeah.
Rhonda: And that's, for me, what I want. Another quick question I wanted to ask you...
Jed: It up-regulates its production. So if you look at RNA levels for Nrf2 or for Keap1, its tether protein that's present in the cytoplasm. After treatment with sulforaphane, you see those levels going up. So 60%...
Rhonda: I think that was a translocation of the nucleus. That was the increase that it was doing.
Jed: Okay, yeah. Unfortunately, I can't comment on that, just don't know.
Rhonda: Yeah, I mean, that's okay. I'll talk about it at another...
Jed: But I'm sure it's true, I believe you.
A critical element of the body’s immune response. Inflammation occurs when the body is exposed to harmful stimuli, such as pathogens, damaged cells, or irritants. It is a protective response that involves immune cells, cell-signaling proteins, and pro-inflammatory factors. Acute inflammation occurs after minor injuries or infections and is characterized by local redness, swelling, or fever. Chronic inflammation occurs on the cellular level in response to toxins or other stressors and is often “invisible.” It plays a key role in the development of many chronic diseases, including cancer, cardiovascular disease, and diabetes.
A protein typically present in the cytoplasm of mammalian cells. Nrf2 can relocate to the nucleus where it regulates the expression of hundreds of antioxidant and stress response proteins that protect against oxidative damage triggered by injury and inflammation. One of the most well-known naturally-occurring inducers of Nrf2 is sulforaphane, a compound derived from cruciferous vegetables such as broccoli.
A result of oxidative metabolism, which causes damage to DNA, lipids, proteins, mitochondria, and the cell. Oxidative stress occurs through the process of oxidative phosphorylation (the generation of energy) in mitochondria. It can also result from the generation of hypochlorite during immune activation.
The observable physical characteristics of an organism. Phenotype traits include height, weight, metabolic profile, and disease state. An individual’s phenotype is determined by both genetic and environmental factors.
An extremely rare genetic disorder in which symptoms resembling aspects of aging are manifested at a very early age. People born with progeria typically live to their mid teens to early twenties. Although the term progeria applies strictly speaking to all diseases characterized by premature aging symptoms, it is often applied specifically in reference to Hutchinson–Gilford progeria syndrome (HGPS).
A truncated version of lamin A protein. Progerin is responsible for Hutchinson-Gilford progeria, a rare, genetic disorder characterized by accelerated aging. Progeria is most often generated by a silent point mutation (C1824T) in the gene.
An isothiocyanate compound derived from cruciferous vegetables such as broccoli, cauliflower, and mustard. Sulforaphane is produced when the plant is damaged when attacked by insects or eaten by humans. It activates cytoprotective mechanisms within cells in a hormetic-type response. Sulforaphane has demonstrated beneficial effects against several chronic health conditions, including autism, cancer, cardiovascular disease, diabetes, and others.
Attend Monthly Q&As with Rhonda
Support our work

The FoundMyFitness Q&A happens monthly for premium members. Attend live or listen in our exclusive member-only podcast The Aliquot.
Sulforaphane News
- Sulforaphane extends lifespan and healthspan in worms via insulin and insulin-like growth factor-1 signaling.
- Paul Saladino, MD explains how we may have overstated the health benefits of plants and especially sulforaphane
- NRF2 much of a good thing (December 2017)
- A pilot trial finds sulforaphane treatment increased glutathione levels in the blood & hippocampus region of the brain in healthy people.
- A new study found sulforaphane (found in broccoli sprouts) improved behavior & social responsiveness in children with autism.





































































