Contents
[HIDE]Nicotinamide adenine dinucleotide (NAD+) is a cofactor (a molecule that assists enzymes in chemical reactions) that plays an essential role in multiple physiological processes, such as energy metabolism, DNA repair, and immune activation. It is necessary for the activity of sirtuins, a class of enzymes involved in longevity, and poly-ADP-ribose polymerases (PARPs), a family of DNA-repair enzymes. Cellular NAD+ production declines with age, however, and its depletion likely drives the onset and progression of multiple age-related conditions such as metabolic dysregulation and neurodegenerative disease.
The human body can synthesize NAD+ from a variety of dietary sources, including tryptophan (an amino acid) and the three forms of niacin (vitamin B3) – nicotinamide (NAM, also called niacinamide), nicotinic acid (NA), and nicotinamide riboside (NR) – commonly referred to as niacin equivalents.[1] Of these three, NA is the primary source of NAD+. These precursors are not equally allocated throughout the body, exhibiting preferential distribution among the blood, brain, gut, and other organs.
Niacin is present in various foods, including beans, milk, meat, and eggs. Nicotinamide mononucleotide (NMN), another dietary precursor of NAD+, can also be found in various foods, such as broccoli, avocado, and beef.[2] Numerous animal and human studies have shown that increasing NAD+, either through the dietary intake of a precursor or via direct supplementation, may be useful in preventing or reversing age-related disease.[3]
Biosynthesis & recycling
The body's predominant source of NAD+ is the NAD+ salvage pathway. Enzymes such as sirtuins and PARPs consume NAD+ molecules, depleting cellular levels and generating nicotinamide (a niacin equivalent) as a byproduct. The salvage pathway converts nicotinamide to nicotinamide mononucleotide (NMN) via the enzyme nicotinamide phosphoribosyltransferase (NAMPT). Through multiple steps, NMN converts to NAD+.[4] It is important to note that this enzyme is subject to feedback inhibition by NAD+ levels, so after reaching a certain level of NAD+, the salvage pathway stops producing NAD+.
Energy sensor
NAD+ is critical for energy generation within cells. In the mitochondria, NAD+ is a cofactor for metabolic enzymes involved in oxidative phosphorylation, a process in which energy production occurs via the transfer of electrons from electron carriers (such as NAD+) to oxygen, forming ATP. Outside the mitochondria, NAD+ is a cofactor for enzymes involved in glycolysis – the production of energy in the form of ATP from glucose.[5]
When cellular energy levels are low, such as during exercising, fasting, or caloric restriction, cellular NAD+ levels rise, and the ratio of NAD+ to its reduced form, NADH, increases, thereby serving as a "sensor" to turn on energy-generating pathways and activate enzymes such as sirtuins.[6] Conversely, the depletion of NAD+ due to DNA damage or aging may result in the suppression of NAD+-dependent ATP generation and a possible cellular energy crisis.

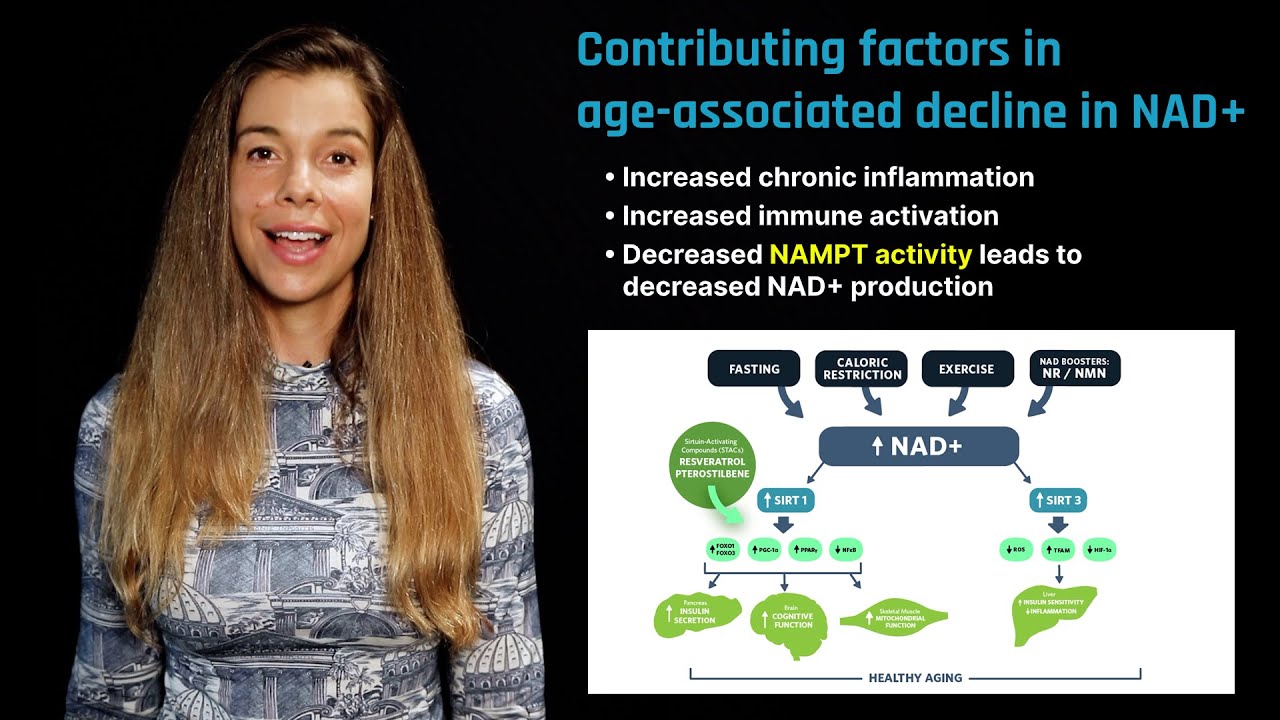
NAD+ status in aging and potential for intervention. Cellular levels of NAD+ increase under conditions of energy stress, such as caloric restriction or exercise; via supplementation of NAD+ precursor molecules, NR and NMN, which "boost" synthesis; or via sirtuin-activating compounds (STACs), such as resveratrol or pterostilbene. NAD+ levels decrease during aging due to increased NAMPT activity, as well as age-related cellular demand for PARP-directed DNA repair, immune function, and inflammation.
Aging
NAD+ depletion may drive the hallmarks of aging, including decreased autophagy, increased DNA damage, increased mitochondrial dysfunction, and dysregulated nutrient sensing.[3] Depletion of NAD+ may predispose organisms to various age-related diseases, including neurodegenerative diseases, metabolic diseases, and cancer.[7] NAD+ levels decrease with age in multiple species, including humans.[8] [9] [10]
In contrast, NAD+ levels increase under conditions that promote greater healthspan or lifespan, such as exercise or calorie restriction.[6] [11] Furthermore, NAD+ restoration has been shown to increase lifespan in lower-level organisms such as yeast and worms, as well as in rodents.[12] [13] In a mouse model of Alzheimer's disease, increasing NAD+ through precursors NR or NMN ameliorated mitochondrial dysfunction and neuronal cell death, thereby slowing cognitive decline.[14] [15]
Taken together, these findings suggest that NAD+ plays a critical role in aging. Specifically, the reduced NAD+ levels commonly observed in aging may arise from decreased synthesis, increased consumption, and increased degradation.[16] Depletion of NAD+ can result in unrepaired DNA damage since PARP-1 requires NAD+ to function. Furthermore, immune cells activated upon injury and inflammation have a high energy demand and may deplete NAD+ pools. Therefore, increased DNA damage and inflammation commonly observed in aging may decrease NAD+ and potentiate aging.[17]
Physiological responses of NAD+
NAD+ is involved in multiple physiological processes such as DNA repair, regulation of inflammation, autophagy, and energy metabolism, all of which play essential roles in regulating healthspan and aging.
DNA damage & DNA repair
As organisms age, accumulation of DNA damage may drive processes of aging and the development of age-related diseases. PARP enzymes, however, detect DNA damage and initiate a repair response by signaling other DNA-repair enzymes. In this way, PARP likely influences longevity. For example, PARP activity in the white blood cells of multiple mammalian species predicts maximum lifespan.[18] Furthermore, findings from a study assessing PARP-1 across multiple mammalian species demonstrated that the higher the PARP-1 activity, the longer the lifespan.[19] The difference in PARP-1 activity between the longest-lived mammals tested (humans) and the shortest-lived mammals tested (rats) was 5-fold. Remarkably, a study that used cell lines derived from centenarians (people older than 100 years) demonstrated that the cells' PARP activity was considerably higher than cell lines from much younger adults. PARP-1 activity has also been correlated with maximum lifespan in mammals.
Skin is particularly vulnerable to the effects of aging. A study that examined human skin samples from 49 people between the ages of 15 and 77 years found that with age, PARP-1 activity increased, but NAD+ levels decreased.[20] Excessive DNA damage has also been shown to reduce NAD+ to 20 to 30 percent of its normal levels.[21] [22] As we age and accumulate more DNA damage, our cells increase their demand for DNA repair. The chronic reduction of NAD+ levels may eventually lead to the depletion of NAD+. The hyperactivity of PARP-1 and subsequent consumption of NAD+ may limit the activity of other NAD+-consuming enzymes, such as sirtuins.[23]
.jpg)
Increases to the NAD+ pool promote sirtuin activity. SIRT1 activation leads to improved insulin function in the liver, improved cognitive function in the brain, and increased mitochondrial biogenesis in skeletal muscle. SIRT3 activation leads to improved insulin sensitivity and lowers inflammation in the liver. Loss of sirtuin activity during aging is associated with altered NAD+ status.[4]
Sirtuin activation
NAD+ is a substrate for the sirtuin proteins, a family of enzymes involved in various metabolic processes. Sirtuins utilize NAD+ to remove specific chemical structures called acetyl groups – a process called deacetylation – from cellular proteins to control transcriptional regulation, energy metabolism, circadian rhythms, DNA repair, and cell survival. As described above, the ratio of NAD+ to its reduced form, NADH, increases under conditions of low cellular energy, switching on sirtuin expression and activity. Sirtuins must respond to dynamic changes in NAD+ to trigger appropriate adaptive responses. Broad loss of sirtuin activity, potentially due to a reduction in NAD+, is typically observed with aging, and many animal models demonstrate that decreased SIRT1 activity may promote the pathogenesis of cardiovascular and neurological diseases.[24] [25]
Mitochondrial function
Mitochondria are classically thought of as the cell's powerhouses because they generate energy in the form of ATP. They are also deeply involved in cellular metabolism and signaling pathways. Mitochondrial dysfunction is a hallmark of the aging process and may lead to reduced energy production, disruption of cellular metabolism, and progression of age-related diseases. Mitochondrial maintenance through mitophagy (selective elimination of defective mitochondria) and mitochondrial biogenesis (synthesis of new mitochondria) is critical to maintaining cellular homeostasis and health.[26]
NAD+ is an important regulator of mitochondrial maintenance. The rise of NAD+, due to fasting, for example, has been shown to induce mitochondrial biogenesis.[27] Furthermore, raising NAD+ levels in old mice can restore mitochondrial function to those of a young mouse.[28]
Circadian rhythm
The circadian system, composed of multiple cellular clocks found in all cells throughout the body, orchestrates the regulation of gene expression that coordinates metabolic programs needed to support bodily functions. NAD+ levels cycle in a 24-hour rhythm, regulated by the core clock genes CLOCK and BMAL1.[29] These genes control the circadian expression of NAMPT, the rate-limiting enzyme in the NAD+ salvage pathway.[30] Furthermore, NAD+ itself can directly regulate circadian rhythm through the activation of sirtuins.[31] [32] Thus, NAD+ is extensively involved in the regulation of the circadian clock, which, when functioning correctly, helps maintain homeostasis and health.
Energy metabolism
NAD+ plays a crucial role in energy metabolism by accepting and donating electrons through the back-and-forth processes of reduction and oxidation – often referred to as redox reactions. These alternating conversions of NAD's oxidized form (NAD+) to its reduced form (NADH) play significant roles in the reactions associated with the metabolism of glucose and fatty acids and the formation of ATP. Since both the oxidized and reduced forms of NAD+ are essential for these linked sets of reactions, cells maintain significant concentrations of both NAD+ and NADH. Without NAD+ and its related molecules, life would cease to exist.[33]
NAD+ boosters & supplementation
Boosting NAD+ levels extends lifespan and healthspan in premature aging animal models, prompting further investigation of NAD+ supplementation in humans to treat and prevent age-related diseases.[7] In particular, two essential NAD+ precursors, nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), have been extensively studied and shown to ameliorate age-associated diseases in animals.1 NR and NMN are well tolerated at high doses and effectively raise NAD+ levels in rodents. Currently, only NR has been shown to raise plasma NAD+ levels and may have beneficial health effects in humans.[34] [35] Generally, cells do not directly take up NAD+, however, and studies in humans regarding the efficacy and safety of NAD+ supplementation by intravenous infusion are lacking.
NAD+ has poor bioavailability. Animal studies have shown that upon ingestion, NAD+ is broken down into the precursor nicotinamide primarily, but also NR and NMN, before being absorbed.[36] While oral bioavailability of NAD+ is low, it is possible that intravenous NAD+ infusion may be used to bypass the digestive system. Unfortunately, research has not identified a mammalian NAD+ transporter. In addition, extracellular NAD+ does not appear to be taken up into tissues, barring the brain and the heart. For example, NAD+ administered via intraperitoneal injection increased NAD+ in some brain regions in mice.[37] Similarly, mice injected with a high dose of NAD+ had increased levels in the heart and protection from cardiac hypertrophy.[38] In-vitro studies have also shown some cell types might be able to transport extracellular NAD+ across the plasma membrane.[39]
A recent pilot study in humans investigated changes in plasma and urinary levels of NAD+ and its metabolites during NAD+ infusion. The study involved 11 healthy men between 30 and 55 who received 750 milligrams of NAD+ over six hours, administered via intravenous infusion. After the first two hours of infusion, the investigators detected no changes in NAD+ or its metabolites (such as nicotinamide, methylnicotinamide, or adenosine phosphate ribose), indicating that the infused NAD+ was entirely removed from the plasma and likely absorbed by tissues. However, after two hours of infusion, NAD+ and its metabolites were detected in the plasma at levels approximately 400 percent above baseline levels, likely indicating tissue saturation.[40] While this study revealed for the first time the potential fate of exogenous intravenous NAD+ in humans, follow-up studies are needed to determine the exact metabolic fate of infused NAD+.
Nicotinamide riboside versus nicotinamide mononucleotide
Levels of NAD+ fluctuate throughout the body's tissues based on cellular consumption and precursor or substrate availability. The highest variation occurs in the small intestine and spleen, and the lowest occurs in the skeletal muscle.
One study used isotope tracers to track NAD+ flux in mice relative to its source.[41] When oral tryptophan was the source, the liver produced NAD+ for local use and broke down into nicotinamide, as described above. Nicotinamide traveled to other tissues, such as the muscles, brain, and gut, which used salvage pathways to convert it back to NAD+.
The study's authors investigated whether oral NR and NMN would travel to other tissues to form NAD+ directly, bypassing the NAMPT feedback inhibition. Using isotope tracers that allowed them to distinguish NAD+ made directly from NR or NMN versus NAD made from nicotinamide, NR, or NMN, they found that a low oral dose of the precursors (50 milligrams per kilogram body weight, mg/kg/bw) produced very low levels of NAD+ made directly from NR and NMN in the liver but not in other tissues. Low levels of nicotinamide-derived NAD+, on the other hand, were found in the kidneys, muscles, and brain, formed from the salvaged nicotinamide in NR and NMN.
A higher oral dose of 200 mg/kg/bw of NR showed no difference compared to a low dose in making direct NAD+ in tissues other than the liver. However, they found more nicotinamide-derived NAD+ in the kidney, muscle, and brain than at a lower dose of 50 mg/kg/bw.
When NR and NMN were given intravenously at varying doses (50 mg/kg/bw and 500 mg/kg/bw), directly produced NAD+ was present in the liver, kidney, and muscle in a dose-dependent manner. However, the only NAD+ detected in the brain was that which was salvaged from nicotinamide, suggesting that neither NR nor NMN crosses the blood-brain barrier. It is noteworthy that a head-to-head comparison of identical doses of injected NR and NMN produced more NAD+ made directly from NR in the liver, kidney, and particularly in the muscle, compared to NMN.
NADH as a source of cellular NAD+
The reduced form of nicotinamide adenine dinucleotide (NADH) plays significant roles in the reactions associated with the metabolism of glucose and fatty acids and the formation of ATP. Few studies have investigated whether supplementation with NADH can increase NAD+ levels; however, some studies have investigated NADH as a potential treatment for Alzheimer's disease. In a study of 25 people diagnosed with mild to moderate dementia, 10 milligrams of oral NADH taken daily for three months elicited no evidence of cognitive improvement among the study participants.[42] In a separate study, however, 12 patients diagnosed with Alzheimer's disease received 10 milligrams of NADH daily for six months. At the end of the study period, the patients who received NADH demonstrated improved scores on the Mattis Dementia Rating Scale, such as better verbal fluency, visual constructional ability, and improved abstract verbal reasoning. While the findings from these studies appear contradictory, the trials' durations may have influenced the outcomes. Still, more studies are needed to determine whether NADH increases cellular NAD+ and if it may improve symptoms associated with Alzheimer's disease.[43]
Conclusion
NAD+ mediates multiple physiological processes, such as energy metabolism, DNA repair, and immune activation. Without NAD+, life would cease to exist. As we age, NAD+ levels fall, driving age progression and age-related diseases. Boosting NAD+ improves lifespan and healthspan in multiple animal models and improves age-related diseases in humans.
Topics related to Aging
view all-
FOXO
FOXO proteins are transcriptional regulators that play an important role in healthy aging. Some FOXO genes may increase lifespan.
-
Sirtuins
Sirtuins play a key role in healthspan and longevity by regulating a variety of metabolic processes implicated in aging.
-
Nicotinamide riboside
Nicotinamide riboside is a precursor of NAD+, a coenzyme necessary for energy production and cellular repair. It is available from food and supplements.
-
Nicotinamide mononucleotide
Nicotinamide mononucleotide is a precursor of NAD+, a coenzyme necessary for cellular energy production and DNA repair. It is available as a supplement.
-
Metformin
Metformin, a drug commonly used to treat type 2 diabetes, may modulate certain aging processes.
-
Caloric restriction
Caloric restriction is the practice of long-term reduced energy intake. It delays the onset of age-related chronic diseases in animals.